Author Guidelines
Jurnal Radiologi Dentomaksilofasial Indonesia (JRDI) accepts any kind of submissions from original research articles, case reports and review articles mainly in the field of Dentomaxillofacial Radiology. Starts from April 2021, JRDI only accepts manuscripts in English in order to reach international readers.
For further guidance, authors should always use and refer to our article templates at the sidebar. Any submissions that did not follow the guideline templates will be returned. The author will be notified and charged for Author Fees if the manuscript is accepted to be published through the editorial's decision. Please fill the Transfer of Copyright Agreement Form and submit it along with your manuscript in the submission process.
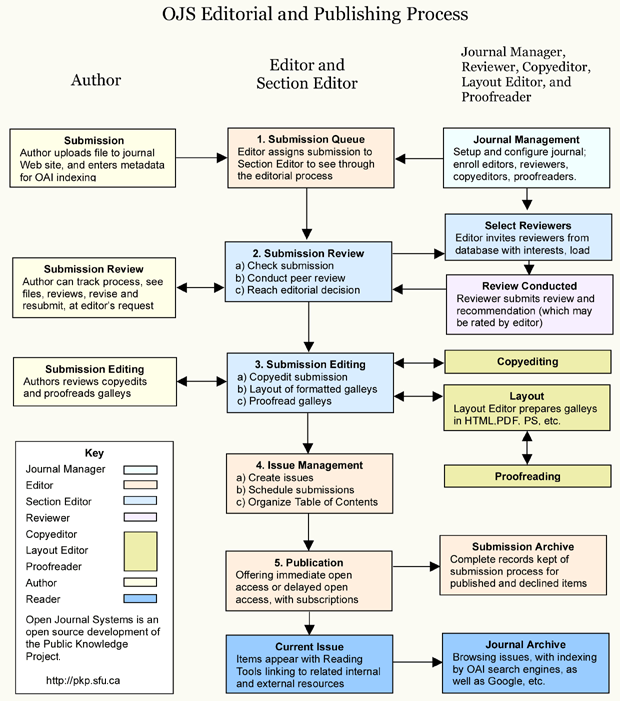
The results of the final layout version of submitted articles could be different from what authors made with the template we provide in the .docx version, due to the flexibility needed by our editors who use different versions of software (JRDI uses Microsoft Publisher as the layout editing software). JRDI uses Open Journal System (OJS) as the journal's main application of admission and publishing with the scheme and general guidelines as follows.
SECTIONS OF THE PAPER
TITLE
- Title does NOT exceed more than 20 words;
- Title uses descriptive sentences associated strongly with the content;
- Title includes author’s complete name, affiliation, complete address for correspondence, telephone number, and email address;
- Title should be short and unambiguous;
- Title should contain all the keywords;
- In descriptive study, the title may include place and period of study. It depends on the essential factors, especially for a result that may NOT be generalizable to other location;
- Placed at the top of the page with justified-aligned, with Sentence Case, NOT underlined or italic except for any terms NOT in Bahasa Indonesia.
ABSTRACT
- Abstract is written BOTH in English and Bahasa Indonesia (if applicable);
- For research report, abstract is written under this structure: Objectives, Methods, Results, Discussion and Conclusion. For any other kind of manuscripts such as reviews and case reports, please refer to our template at the sidebar;
- Abstract is written in section, does NOT exceed 250 words, summarizes the major aspects of the entire paper in the following prescribed sequence: (A) The question(s) you investigated (or purpose), state the purpose very clearly in the last sentence of the Introduction section, (B) The experimental design and methods used (Methods section), (C) The major findings including key quantitative results, or trends, along with a brief summary of your interpretations (Results And Discussion section), (D) Clearly state the implications of the answers the entire research gave you, NOT the results of the statistical analysis (Conclusion section);
- All acronyms or abbreviations in the abstract are defined when first mentioned, and the acronyms or abbreviations are written in parentheses afterward;
- Abstract contains 3–5 keywords.
INTRODUCTION
- Establish the context of the work being reported by discussing the relevant primary research literature (with citations) and summarizing our current understanding of the problem you are investigating;
- State the purpose of the work in the form of the hypothesis, question, or problem you investigated;
- Briefly explain your rationale and approach and, whenever possible, the possible outcomes your study can reveal;
- Do NOT use bullets or numberings list in this section. If so, turn it into a paragraph.
MATERIALS AND METHODS
- FOR FIELD STUDIES ONLY: Describe the site where your field study was conducted;
- FOR LABORATORY STUDIES: You need NOT report the date and location of the study UNLESS it is necessary information for someone to have who might wish to repeat your work or use the same facility;
- Describe your experimental design clearly;
- Describe the procedures for your study in sufficient detail that other scientists could repeat your work to verify your findings (as their citation);
- Describe how the data were summarized and analyzed. The information should include statistical software used, how the data were summarized (means, percentage, etc.) and how you are reporting measures of variability (SD, SEM, 95% CI, etc.) which data transformations were used (e.g., to correct for normal distribution or equalize variances) statistical tests used concerning the particular questions, or kinds of questions, you address any other numerical (e.g., normalizing data) or graphical techniques used to analyze the data what probability (a priori) was used to decide significance; usually reported as the Greek symbol alpha;
- Should have references citation if needed;
- Mention approval from Ethical Committee;
- Do NOT use bullets or numberings list in this section. If so, turn it into a paragraph.
RESULTS
- Objectively present your key results, without interpretation, in an orderly and logical sequence using both text and illustrative materials (Tables and Figures);
- Report your results to provide as much information as possible to the reader about the nature of differences, or directionality, or magnitude;
- Organize the results section based on the sequence of Table and Figures you'll include;
- The body of the Results section is a text-based presentation of the key findings which includes references to each of the Tables and Figures;
- Statistical test summaries (test name, p-value) are usually reported parenthetically in conjunction with the biological results they support;
- Avoid devoting whole sentences to report a statistical outcome alone;
- Avoid the use and over-use of the word "significant";
- Present the results of your experiment(s) in a sequence that will logically support (or provide evidence against) the hypothesis, or answer the question, stated in the Introduction;
- Report negative results;
- Always enter the appropriate units when reporting data or summary statistics;
- Do NOT use bullets or numberings list in this section. If so, turn it into a paragraph.
DISCUSSION
- Interpret your results in light of what was already known about the subject of the investigation, and to explain our new understanding of the problem after considering your results;
- Fundamental questions to answer in Discussion section include: (1) Do your results provide answers to your testable hypotheses? If so, how do you interpret your findings?, (2) Do your findings agree with what others have shown? If NOT, do they suggest an alternative explanation or perhaps an unforeseen design flaw in your experiment (or theirs?), (3) What is our new understanding of the problem you investigated and outlined in the introduction?;
- You must relate your work to the findings of other studies - including previous studies you may have done and those of other investigators;
- Do NOT introduce new results in the Discussion;
- Do NOT use bullets or numberings list in this section. If so, turn it into a paragraph.
CONCLUSION
- Conclusion must answer what is stated in Objectives of the manuscript;
- The outcome of the statistical analysis is NOT a key result, but rather an analytical tool that helps us understand what our key result is;
- Conclusion is the key result, the most important outcome of your work;
- Do NOT simply summarize the points already made in the body — instead, interpret your findings at a higher level of abstraction;
- Make the Conclusion interesting and memorable for readers.
ACKNOWLEDGMENTS
Acknowledgments may be stated only when it relates to any financial supports, grants or other funding schemes by mentioning the individual or institution concerned. Acknowledgments are always brief and never flowery. Otherwise, you may write down this section as: None, if there is no acknowledgment.
FOOTNOTES
Footnotes contain conflict of interest disclosure by the authors and ethical clearance statement related to human/animal rights (if applicable). All articles that are published in this journal MUST be accompanied by a conflict of interest statement. If all authors declare that they have no conflict of interest, you may write down this section as: All authors have no potential conflict of interest to declare for this article. For human or animal studies, provide the ethical clearance statements by stating the ethics approval letter number, or disclose whether all procedures that followed were in accordance with the ethical standards of the responsible committee. If the studies do not contain any human or animal subjects, you may write down this section as: This article does not contain any studies with human or animal subjects performed by the any of the authors.
REFERENCES
- The references should NOT dated more than past 10 years;
- 80 percent of the references should come from scientific journals with the rest comes from textbooks or others;
- References must be up to 15 citations in total.
- Do NOT label this section "Bibliography". A bibliography contains references that you may have read but have NOT specifically cited in the text. Bibliography sections are found in books and other literary writing, but NOT scientific journal-style papers;
- The references citation is in numeric order according to the first mention in the text, under the style of VANCOUVER SUPERSCRIPT referencing system;
- Vancouver is a numbered referencing style commonly used in medicine and science, and consists of: citations to someone else's work in the text, indicated by the use of a number, a sequentially numbered reference list at the end of the document providing full details of the corresponding in-text reference. It follows the rules established by the International Committee of Medical Journal Editors, now maintained by the U.S. National Library of Medicine. It is also known as Uniform Requirements for Manuscripts submitted to Biomedical Journals;
- References are listed in numerical order, and in the same order in which they are cited in the text. The reference list appears at the end of the paper;
- Begin your reference list on a new page and title it 'References.';
- The reference list should include all and only those references you have cited in the text. (However, do NOT include unpublished items such as correspondence);
- Use Arabic numerals (1, 2, 3, 4, 5, 6, 7, 8, 9);
- Abbreviate journal titles in the style used in the NLM Catalog;
- Check the reference details against the actual source;
- Be consistent and avoid repetition with your referencing style across the document;
- We STRONGLY recommend you to use any kind of reference softwares, e.g. Mendeley®, to help sorting all your citations easier and more neatly.














































